COVID-19 Vaccines Arrive
In recent weeks, the rollout of several COVID-19 vaccines has been a beacon of hope for many as the number of global cases continues to rise.
On November 9, American pharmaceutical company Pfizer and its German partner company, BioNTech, announced that its vaccine had a 90% efficacy rate in clinical trials. Just a week later, on November 16, American biotechnology company Moderna followed suit with a vaccine that was 94.5% effective in trials. Foreign vaccines, such as AstraZeneca in the U.K., Sinovac in China, and Sputnik V in Russia, have been produced with similar success rates. While vaccines typically take several years to be created and approved, these have only taken approximately eight months to develop, making them the fastest vaccines ever produced.
On December 10, the Food and Drug Administration (FDA) granted the Pfizer-BioNTech vaccine Emergency Use Authorization (EUA), officially approving it for distribution in the United States for patients 16 and older. EUA provides an alternative, more rapid form of approval during a public health emergency. While many previous vaccines have taken at least a year to be fully FDA-approved, the FDA approved the Pfizer vaccine in little over a month.
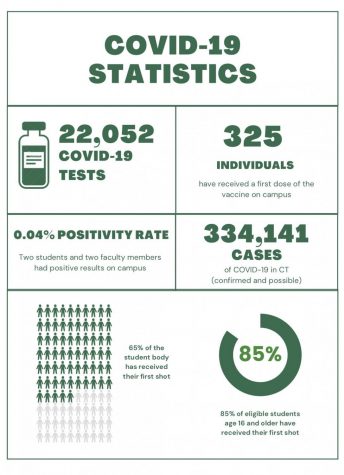
The first doses of the Pfizer vaccine were delivered to 145 hospitals on December 14. The U.S. government is paying approximately two billion dollars for 100 million doses of the vaccine and has the option to purchase up to 500 million more doses in the coming months. Each individual is supposed to receive two doses of the vaccine three weeks apart for the Pfizer vaccine and four weeks apart for the Moderna vaccine. Moderna’s vaccine still awaits EUA approval; the FDA will hold a hearing on December 17.
The successful vaccines pave way for herd immunity, meaning that the general population can be protected against the virus whether or not everyone takes the vaccine. Dr. Jared Zelman, medical director, said, “If we can get to 70% or 80% participation in the United States, or if not the world, then it’s possible that the degree of infectiousness can drop below one and we [can] succeed in eliminating this disease.”
The vaccines from Pfizer and Moderna use messenger RNA (mRNA) to stimulate an immune response; these are the first mRNA vaccines ever approved for use in humans. Rather than using a dead or weakened version of the virus, these vaccines contain mRNA, which causes the person’s cells to produce spike proteins. These spike proteins trigger the body to produce antibodies, which then recognize and fight COVID-19, should the vaccinated person become infected.
When the school will receive vaccinations is uncertain. States are prioritizing frontline healthcare workers and nursing home residents when distributing the vaccine. Next in line for vaccinations will likely be essential workers, which may include teachers, and those more vulnerable to the virus, such as the elderly and those with pre-existing conditions. The general public is not projected to receive the vaccine until mid-2021. For this reason, Dr. Zelman does not foresee any changes to the school’s COVID-19 restrictions until the 2021-2022 academic year.
Despite this, the prospect of a vaccine is a light at the end of the tunnel for those who want to return to normal life on campus. Jack Johnson ’22, who works as an Emergency Medical Technician with the New Canaan Emergency Medical Services, said, “I am beyond proud of how well our student community handled the pressures of restrictions on social and interpersonal relationships and connections.”
The New York Times created this interactive activity to help people estimate when they might receive the vaccine.